
Canola meal as an alternative protein source for dairy cows
This is a project sponsored by the Canola Council of Canada that aims to assess whether canola meal (CM) has the potential to replace soybean meal (SSBM) to lactating dairy cows. So far we have been able to demonstrate that replacing SSBM with CM increases dry matter intake (DMI), yields of milk and true protein, and depress milk urea nitrogen (MUN), ruminal NH3, and branched-chain volatile fatty acids (BCVFA) concentration, indicating an improvement in Nitrogen utilization (Broderick, Faciola & Armentano 2015). The next step is to better understand the reasons that make CM a better Nitrogen source to dairy cows. Another finding from our canola research is that CM nutritional composition varies among production sites in Canada. Twelve different canola crushing plants were surveyed over four years and differences in rumen undegraded protein (RUP) ranged from ~40-50% of crude protein, which could impact dairy production (Broderick et al., 2016).
So far, we have shown that both in vitro and in vivo this RUP differences do not affect ruminal metabolism and nutrient flow Paula et al. (2017) and Paula et al. (2018).
Omasal sampling technique
For the past several years I have worked on a research method commonly referred to as the “omasal sampling technique”, which allows in vivo measurement of ruminal protein degradation and escape, microbial protein and NAN flow out of the rumen, ruminal digestion of NDF, starch, and OM, as well as fatty acid flow out of the rumen. This technique is an alternative to duodenal sampling, which is more expensive, more invasive, and harmful to the animals; furthermore, duodenal sampling is less reliable because samples may be contaminated with abomasal secretions. I have conducted omasal sampling in numerous experiments in the U.S. and abroad, sampling dairy cows, beef cattle, goats, and sheep. I have also trained over twenty graduate students and visiting scientists from Argentina, Brazil, Canada, Chile, China, Italy, Mexico, Norway, Spain, Thailand, Turkey, and the USA. Because this technique can be used in a broad range of animals, is less invasive and allows direct in vivo determination of microbial growth, digestion, and flow of nutrients and, because very few people in the world have mastered it, I consider this technique to be my contribution of greatest impact in animal nutrition.
One of the goals of our laboratory is to continue to disseminate this methodology worldwide so we can collect more data that will improve our understanding of ruminant nutrition and contribute to currently used models such as the NRC. For example, the 2001 NRC protein model is based on protein degradation and escape, estimated using in situ data, and microbial protein flow from experiments with animals cannulated in the abomasum or duodenum. In situ protein degradation methodology is of limited value because of several inherent issues, such as: physical restriction of feeds within the bag that limit microbial digestion; imprecise quantification of microbial contamination of the undigested residues; and the assumption that proteins, peptides, and amino acids in the soluble fraction are completely degraded. Duodenal sampling has been the principal method applied for assessing dietary and animal factors influencing microbial protein formation in the rumen. Flow estimates from duodenal sampling have been variable; for example, ruminal OM and starch digestibility obtained using duodenal sampling have been unrealistically low and even negative. Also, unrealistically high or even negative proportions of hindgut digestion of total NDF digestion have been reported. I am confident that omasal sampling has the potential to improve our understanding and enhance current nutritional models.
A couple of recent peer-review publications using this technique are Faciola and Broderick (2014) and Paula et al. (2018).
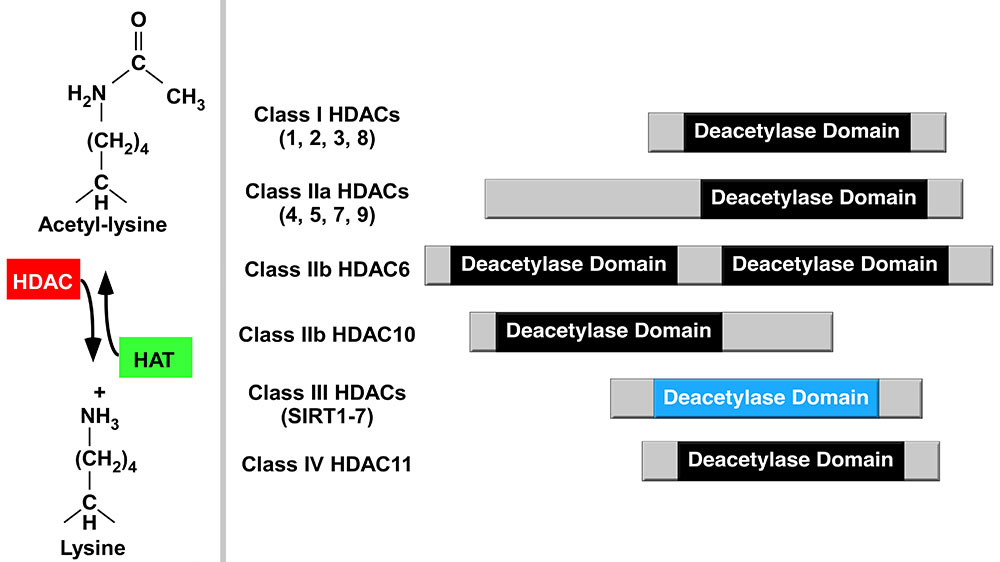
Nutritional regulation of inflammatory gene expression
Inflammation impacts cattle production as well as puts an economic burden on producers. Our lab, in collaboration with the Ferguson Lab, is interested in the roles nutrients play on histone deacetylases (HDAC) activity, inflammatory signaling, and inflammatory gene expression. Acetylation of lysine residues on histone tails within chromatin provides an important mechanism for regulating gene expression. Histone acetyltransferases (HATs) and HDACs, which govern lysine acetylation, have emerged as critical regulators of inflammation and have studied as epigenetic modifiers. One of our goals is to dissect the mechanisms by which different nutrients regulate chromatin-dependent signal transduction and inflammatory gene expression in different bovine tissues.
A few recent peer-review publications using this technique are: Silva et al. (2018), Romanick et al. (2018), and Silva et al. (2018).

Alternative Feedstuff for Livestock
Glycerin (or glycerol) is an organic compound from the alcohol group that is fluid at room temperature, odorless, hygroscopic, viscous, and have a sweet taste. It is usually found asa component of triglycerides and phospholipids and has great potential as energy source for livestock. Glycerin is also an important by-product of biodiesel production, which makes it cheaply available in certain areas. This project was developed based on three independent experiments with the objectives of: 1)- Evaluate the effects of partially replacing corn with glycerin on ruminal fermentation using a dual-flow continuous culture system; 2)- Assess the effects of partial replacing corn with glycerin on performance and carcass characteristics of beef cattle finished in feedlot; 3)- Identify the influence of glycerin inclusion on in vitro ruminal fermentation, total gas, and methane productions in finishing beef diets. Findings from this project have been published in two peer-review articles and another manuscript is in its final stages of preparation. So far we have been able to demonstrate that glycerin inclusion in beef cattle diets may change in vitro ruminal fermentation, by increasing total volatile fatty acids, and propionate concentration, indicating an improvement on glycogenic potential (Benedeti et al. 2015). Moreover, results from the in vivo study suggest that glycerin may be included in finishing beef diets at levels up to 15% without impairing performance and carcass characteristics (Benedeti et al. 2016). We have also evaluated the effects of glycerin on gas production kinetics and enteric greenhouse gas emissions (Benedeti et al. 2018).
Moreover, we are interested in evaluating alternative forages for ruminants. A couple of recent peer-review publications evaluating alternative forages for ruminants are Silva et al. (2017) and Silva et al. (2018).

Enhancement of the dual-flow continuous culture system
Dual-flow continuous culture system is an in vitro technique that simulates rumen digestion in which different feeds and artificial saliva are mixed with fresh rumen fluid. In this system, pH, temperature, anaerobiosis, and flow rates (liquid and solid) are tightly controlled. This technique has been widely used to evaluate the effect of complete diets and individual feed ingredients on ruminal digestion, fermentation, microbial protein synthesis, and nutrient flow. The advantages of this technique are: 1) ability to test a large number of treatments 2) ability to test high levels of one specific ingredient, 3) reduced experimental time, 4) reduced amount of total feed used, 5) less animal use, and 6) lower cost when compared to in vivo experiments.
We can measure the chemical composition of different forages, feed ingredients, feed additives, and TMRs (total mixed rations), as well as apparent and true digestibility, ruminal fermentation, microbial protein growth, and nutrient flow (NH3, amino acids, peptides, proteins, short, medium, and long-chain fatty acids, carbohydrates, vitamins, and minerals). We can also measure methane (CH4) an important greenhouse gas. The results of this studies provide livestock producers and extension agents with scientifically sound information on the nutritional value of different ingredients and their potential as feedstuff for ruminants. Therefore, producers and extension agents can have more tools to better formulate diets to meet animal’s nutritional requirements, which can lead to better production and higher profits. These management decisions may also have a positive impact on the environment because land and natural resources may be more efficiently used.
Recent peer-review publications using this system include: Benedeti et al. (2015), Silva et al. (2016), Amaral et al. (2016), Silva et al. (2017), Paula et al. (2017), Dai et al. (2017), Silva et al. (2018), Brandao et al. (2018a), and ,Brandao et al. (2018b).
Suppressing ruminal protozoal population
Another area of research that we have explored was attempting to change the ruminal microbial population – notably rumen protozoa, with the goal of improving Nitrogen utilization. This work was partially done in collaboration with Dr. Alexander Hristov from Penn State University. At the time of these studies, our lab had no previous experience working with ruminal protozoal counting techniques, which we developed using an adaptation of Dr. Burk Dehority’s procedure from The Ohio State University. The results of those experiments were published in the following articles: Faciola et al. (2013), Faciola and Broderick (2013), and , Faciola and Broderick (2014).
We hypothesized that successful partial suppression of the protozoal population would improve Nitrogen utilization without being harmful to carbohydrate fermentation, thus enhancing animal performance. We evaluated several doses of lauric acid (LA, C12:0) both in the diet and also when dosed straight into the rumen, we also evaluated sodium laurate and finally coconut oil as a potential rumen protozoa suppressant agent for practical conditions. When dosed straight into the rumen LA and sodium laurate had a potent anti-protozoal activity, reducing rumen protozoa population in 90% within just two days; however, when mixed into the diet they were not quite effective because the dose necessary to reduce rumen protozoa also reduced dry matter intake and consequentially milk yield. Another interesting finding from this work was that it takes 12 days for ruminal protozoal population to re-establish itself in the rumen after LA withdrawing from the diets.
Another study (Paula et al. 2016) evaluated the effects of phenolic compounds on ruminal protozoa population in water buffaloes.